Post by Holly Parrish, undergraduate in Psychology and minor in Interdisciplinary Neuroscience at Portland State University. Holly volunteers as a clinical research intern for the Nuvox2 study in a clinical research lab at the Center for Neurosciences Tucson in Arizona.
My name is Holly Parrish, and I am a senior at Portland State University. My major is Psychology, and I’m pursuing a minor in Interdisciplinary Neuroscience. I was lucky enough to be invited to participate in a clinical drug trial for glioblastoma multiforme at the Center for Neurosciences Tucson in Arizona.

LEARN MORE: Glioblastoma Multiforme
LEARN MORE: Clinical Research Program @ Center for Neurosciences Tucson
I have been working to recruit patients who qualify for our study and meet the complex inclusion/exclusion requirements. I do this by meticulously combing through patient files looking for diagnoses, critical events, surgeries, or past medications that could impact participation. Generally, our patient pool comes from Primary Care Providers and Emergency Room Providers throughout the Southwest Arizona area.

Placed in a lab!
The Center for Neurosciences Tucson treats epilepsy, pediatric migraine, and many forms of brain cancer. I am able to work with Dr. Badruddoja, MD, a Board-certified Neurologist with fellowship training in Neuro-Oncology. Neuro-oncology is a medical specialty that focuses on the effects of cancer on the nervous system, including the brain, spinal cord, and peripheral nerves. This can also include treating the neurologic complications of cancer such as seizures, peripheral neuropathy, and quality of life issues.
Clinical research in people involves running experimental trials in several “phases” (I, II and III).
Once initial tests in Phase I establish the safety of a treatment, the Phase II clinical trials test the efficacy, or effectiveness, of the therapy on a larger number of participants. These trials can last several months to, for some studies, up to five years. The decision to move forward into Phase III clinical trials is made during the Phase II trials based on the outcomes. Only about 1/3 of trials progress past Phase II.
LEARN MORE: NIH Clinical Research Trials and You
Dr. Badruddoja’s special interest is in the treatment of malignant glioma, and he has been the Principal Investigator (or P.I.) on multiple Phase II glioblastoma multiforme clinical trials. He is also a Principal Investigator on Alzheimer’s disease trials.

LEARN MORE: So You Want to Be a Principal Investigator
LEARN MORE: Foundations of Neuro-Oncology: A Multidisciplinary Approach
LEARN MORE: Therapeutic Options in Neuro-Oncology
LEARN MORE: Phase II study of bi-weekly temozolomide plus bevacizumab for adult patients with recurrent glioblastoma
LEARN MORE: Donanemab in Early Symptomatic Alzheimer Disease
What is glioblastoma multiforme?
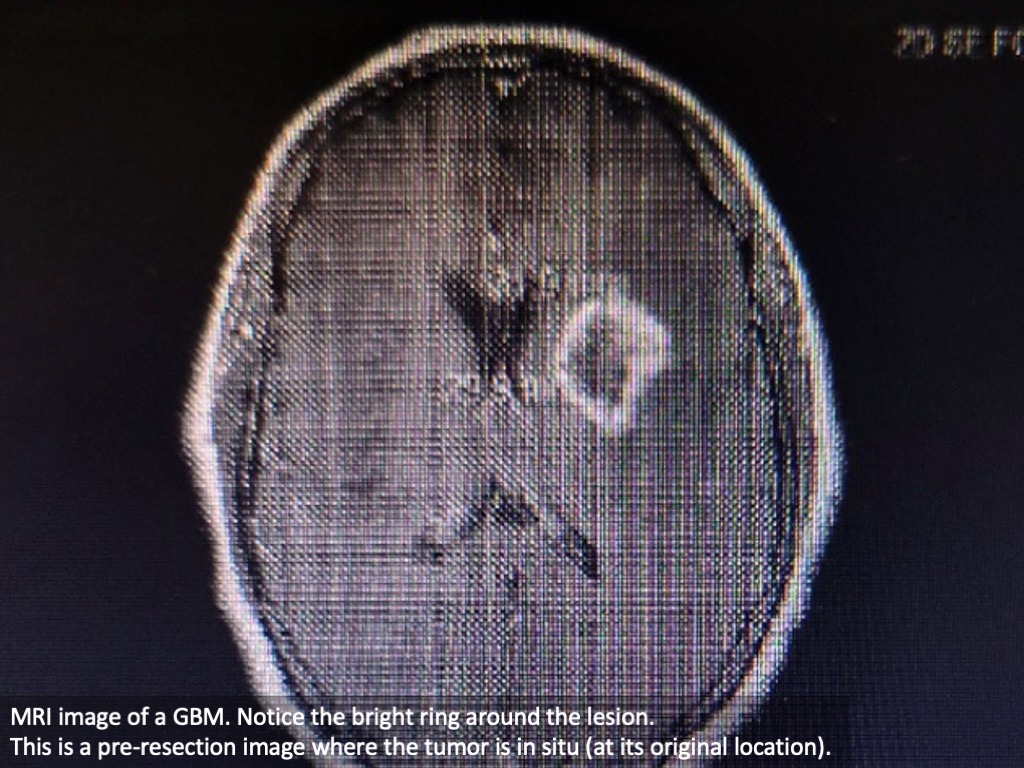
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor. It has a 5-year survival rate of only 7.2%. It is protected by the blood brain barrier and poses treatment challenges outside the standard treatments of surgical resection and chemoradiotherapy.
Chemoradiotherapy is a common cancer treatment that combines chemotherapy drugs and radiotherapy treatment (delivery of radiation). The goal is to achieve the maximum negative effect on the tumor. The “chemo” portion of our therapy involves Temozolomide, a drug that stops cancer cells from dividing and growing. The “radio” portion can include radiation waves which are similar to X rays to kill the cancer cells. The study I was involved in looked at improving the impact of the radiation treatment.
LEARN MORE: Chemoradiotherapy in Cancer Treatment: Rationale and Clinical Applications
The blood brain barrier is an actual physical barrier formed by tight membrane junctions between the cells that make up the capillaries in the brain. They form a barrier that keep many molecules out of the brain. This is helpful for restricting access to dangerous drugs, or disease organisms, but it can make it challenging to deliver enough of the large chemotherapy drugs to the tumor.
Drugs can loosen this barrier, and so can radiation. However, the tightness of that barrier is one reason that therapeutic drug-infused Gliadel wafers are left in the brain after a tumor has been removed.
LEARN MORE: Gliadel wafers in the treatment of malignant glioma: a systematic review
The brain tumors in glioblastoma grow from glial cells known as astrocytes and oligodendrocytes. These glia normally protect the brain, and contribute to brain function, but in glioblastoma they are growing out of control.

LEARN MORE: Glioblastoma multiforme: An overview of current therapies and mechanisms of resistance
LEARN MORE: Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease
LEARN MORE: New advances on glial activation in health and disease
LEARN MORE: What about the glia?
There are several ways to describe tumors.
The word resect means to remove by surgery. Some tumors are easily reached for surgical removal, and their removal doesn’t impact any other brain structures. These are called resectable tumors.
Non-resectable tumors, in contrast, are not easily reached due to their location, the presence of other structures, blood supply, and/or size. Categorization is generally made by a surgeon after imaging, but sometimes a tumor can surprise and upend a plan when visualized directly during surgery.
If the tumor is safely resectable, surgeons remove as much as possible and then after a recovery period start chemoradiotherapy. In this study, they are adding an investigational drug that enhances the uptake of oxygen throughout the body and is theorized to help the radiation portion of the treatment. I cannot tell anything further about the trial, as I have been asked not to disclose any further information.
There are many reasons why a sponsor of a clinical trial might want secrecy.
There can be intellectual property being tested, such as a special drug delivery system or device. Proprietary methods that belong to a company might be involved, or rival companies might try to gain insider knowledge in order to beat them to the market. If a clinical trial suggests that a drug has promise, there is potential for significant financial reward. The clinical research industry is very competitive and closed mouth because the person sitting behind you at the café might take what you are saying and design a study around it, get funding, and beat you to publication. It’s a cutthroat world!
Behind the scenes: Drug trials
The co-investigators in a study are all the staff involved outside of the Principal Investigator. The people I worked with were nurses, social workers, nurse practitioners, phlebotomists, and administrators.
One thing that surprised me is how tightly controlled every aspect of the study is.
The study has to be re-creatable EXACTLY so that the results can be tested by other researchers, and those results can be depended on to support further work. The study I am working on involves a drug known as Nuvox2. Previously, there was a Phase I trial that had different exclusions for prior chemotherapy drugs, and a lower dose of the investigational drug.
Phase I of the Nuvox2 trials revealed a correlation between drug treatment and slower tumor progression, with minimal side effects.
In clinical research correlation does not equal causation. Just because it “appears” that a drug worked, a variable not accounted for in the original trial design might play a role. By moving to Phase II, researchers are using higher doses and a larger group of participants. They also adjusted the trial design and what were/weren’t exclusionary factors in order to include a greater spectrum of patients.
LEARN MORE: NuvOx Pharma Announces Issuance of a New Patent
The study I worked on was double-blind, which means that each patient consented to the possibility of receiving a placebo, or a non-therapeutic substance like normal saline for IV use. The other possibility is that they would receive the drug, which in this case is thick and cloudy.
LEARN MORE: Double-blind study
LEARN MORE: FAQs About Clinical Studies
Because of this, researchers had to be careful about who mixed the drugs, how they were packaged, and what was visible to the patient. For our lab, a computer randomized the patients, and a pharmacist came in from another department to prepare the IV infusion. The only thing that this person knew was that one substance was clear, and the other cloudy. Amber tubing was used on all the IV equipment, and in places along the line that lacked the amber coating the pharmacist applied white silk tape so the fluid was not visible to either the investigators or the patients during administration.

Bravery of trial participants
One thing that struck me as I was present for the consenting process for the trial is how brave these people are. They know that they might not receive a potentially life saving treatment, that outcomes are bad, that treatment with or without the investigational drug is going to be rough, and that their symptoms are going to be uncomfortable.
The four people that enrolled in the study while I was working at the lab were 35, 39, 42, and 58 years old. There was a sense of loss that I had rarely encountered. When they spoke of the trial, it was with an eye to the future. They were completely aware that the outcome of this trial might not even be known before they pass from GBM.
They are invested in helping future patients that they have never met, and never will.

They know that this trial isn’t for them, it’s for the generations of people who will fight GBM in the future. For patients in this position, this is HOPE. It is a way to give back. It helps them find meaning in a time of great turmoil and pain. This is who they really mean when they talk about HEROES that fight cancer.
This is challenging work
When I was invited to participate in this research, I didn’t have a full understanding of what my role would be. I had anticipated “lab only” participation which keeps a distance between investigator and patient. I didn’t expect “patient facing” participation where I was present in the room when the diagnosis was delivered.
LEARN MORE: Clinical Trials and Clinical Research: A Comprehensive Review
LEARN MORE: “Like a nurse but not a nurse”: Clinical Research Practitioners
Looking back, I realize that it was the first time they had had a research intern.
I wish that I had been more clearly prepared for the first time I stood in a room and both the patient and I learned that the visit was regarding terminal brain cancer, and not a simple migraine or loss of words due to a stroke. I would strongly encourage anyone looking at clinical research to be fully aware of the patient facing portion of the work, and the impact that being present in a person’s most vulnerable moment can have on you when you are unprepared, or inexperienced with terminal diseases.
Our patients were treated like royalty when they came for their thrice-weekly treatments. One patient, (who gave me permission to share his story) became fixated on food. He was having a treatment and his wife had left for some errands. He ended up convincing the closest Panda Express that they forgot his catering order, and that he had people waiting on a buffet. The nurses and I both thought that Panda Express would explain that no order was in their system, and we would have snacks and move on with the treatment. Forty-five minutes later Panda Express delivered a buffet for 10 to the infusion lab at the clinic. They were so sorry for messing up this patient’s order. The manager, who was so contrite, refused any payment at all when the patient’s wife explained the whole circumstance. This is one of the things that his wife will remember with a smile once her husband has passed. She will have this memory of his pop-up buffet while she did their shopping to share with their children and grandchildren.
As I near the end of my internship, one of the patients has died, and another has had to drop out of our study due to side effects. I am saddened and relieved to be near completion of the term, and plan to use this experience to help others understand their cancer experience in my future counseling practice. The toll that this kind of work takes on the clinicians and support staff is staggering. It takes a special group of people to knowingly walk through this.

LEARN MORE: Moral Stress and Job Burnout Among Frontline Staff Conducting Clinical Research
LEARN MORE: The Patient-Oriented Clinician-Researcher: Advantages and Challenges
In closing
This was an incredible opportunity.
I am so honored to have been selected to participate in this study. I found out about the Interdisciplinary Neuroscience minor during my senior year at PSU. Luckily, I had fulfilled many of the requirements and was able to include neuroscience in my concentration of studies. I plan on working as a Clinical Mental Health Counselor with an emphasis on the neuroscience of behavior. I think that if you are interested in the brain and how it impacts behavior and perception you should definitely consider this minor.
LEARN MORE: Interdisciplinary Neuroscience minor at Portland State University