Post by Olivia Monestime, undergraduate in Biology pursuing an Interdisciplinary Neuroscience minor at Portland State University. Olivia is a BUILD EXITO scholar working in Dr. Nora Gray‘s research lab at the Portland Veterans Administration Medical Center.

Am I a scientist?

I wasn’t obsessed with science as a kid.
The only scientist I knew was a neighbor who happened to be a rocket scientist. In my young mind, all scientists were geniuses, and he fit that mold perfectly. He would lead science experiments and build rocket launchers for his kids’ birthdays.
But I was never one of those kids eager to launch toy rockets.
I took the major Biology and Chemistry science courses required in high school, but never opted to venture into different science or STEM-related classes. I had other things on my mind at the time.
It wasn’t until my first year of college that I realized my true passion: asking questions. This love for questioning eventually intertwined with my fascination for neuroscience, and I discovered my place in science.
“Everyone is a genius. But if you judge a fish on its ability to climb a tree, it will live its whole life believing that its stupid.”
― Albert Einstein
LEARN MORE: Why Exposure to Science During Childhood Shapes Science Literacy and Comprehension
LEARN MORE: Misconceptions about Science and What it Means to “do” Science
LEARN MORE: The Stereotypical Scientist
How I got into research
I joined the BUILD EXITO program at Portland State University a year late during the pandemic and virtual learning. Despite that, I was driven to make the most of my opportunity. I wouldn’t truly come to find out how valuable that decision was until I was placed in Dr. Nora Gray’s research lab at the Veterans Affairs Hospital in my second year. The main focus in the lab is looking into the effects of different agents on the function of neurons with Alzheimer’s genotypes.
Juggling my Senior year with a new research placement worried me a little bit, but I was excited to be able to do things in the lab that were relevant in my specialized classes in neurobiology and neuroscience.
I stayed on a primary degree pathway of Biology, but I love the brain from all angles, and decided to double-minor in Psychology and Interdisciplinary Neuroscience. I immediately decided to add the neuro minor to my degree when it became available. Dr. Bill Griesar and Jeff Leake’s creative integration of art into the curriculum further ignited my passion for neuroscience. Exploring different modalities of learning opened my eyes to the complexity of the brain.
“There’s only one difference between art and science. In science, the Universe is in control. In art, you are.”
― Harry Kroto
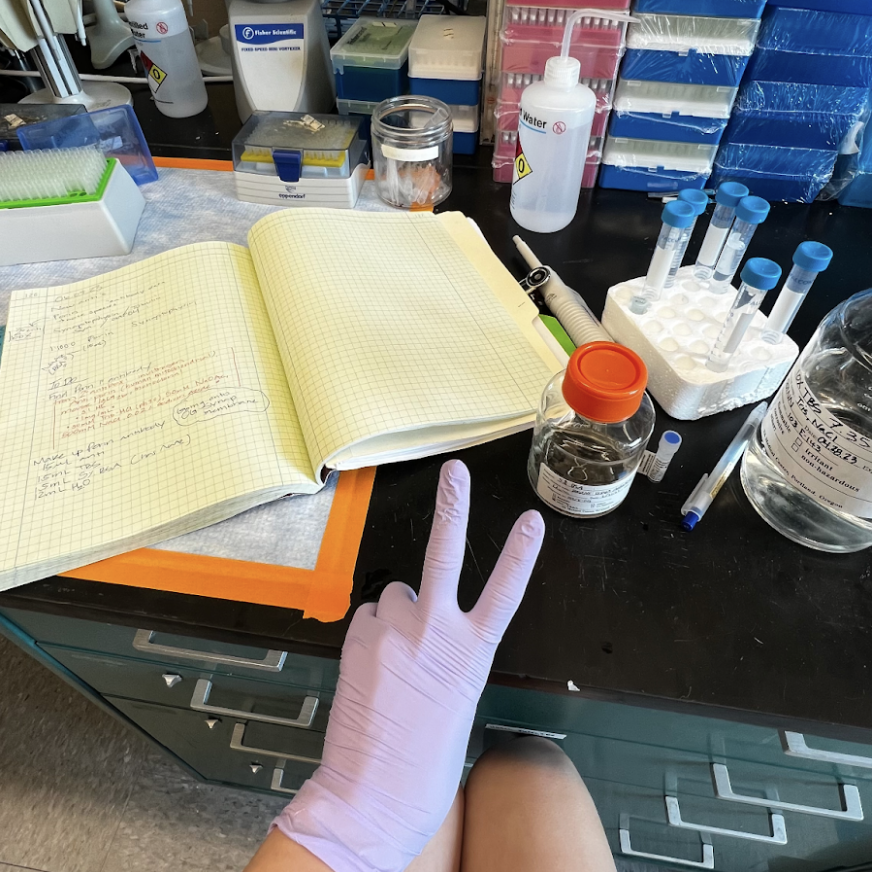
Alzheimer’s Disease (AD) is a neurodegenerative disease that usually becomes apparent after age 65 but in 9% of cases, occurs before then in a form of early onset dementia. Dementia, which can result from a multitude of injuries to the brain, often progresses into AD. Alzheimers affects 1 in 9 people over the age of 65 in the US, but that number fluctuates both regionally and across races. As of right now, the cause and cure of this neurodegenerative disease are relatively unknown.
LEARN MORE: Dementia
LEARN MORE: Alzheimer’s Disease Statistics
LEARN MORE: Alzheimer’s Disease Facts and Figures
LEARN MORE: What Happens to the Brain in Alzheimer’s Disease?
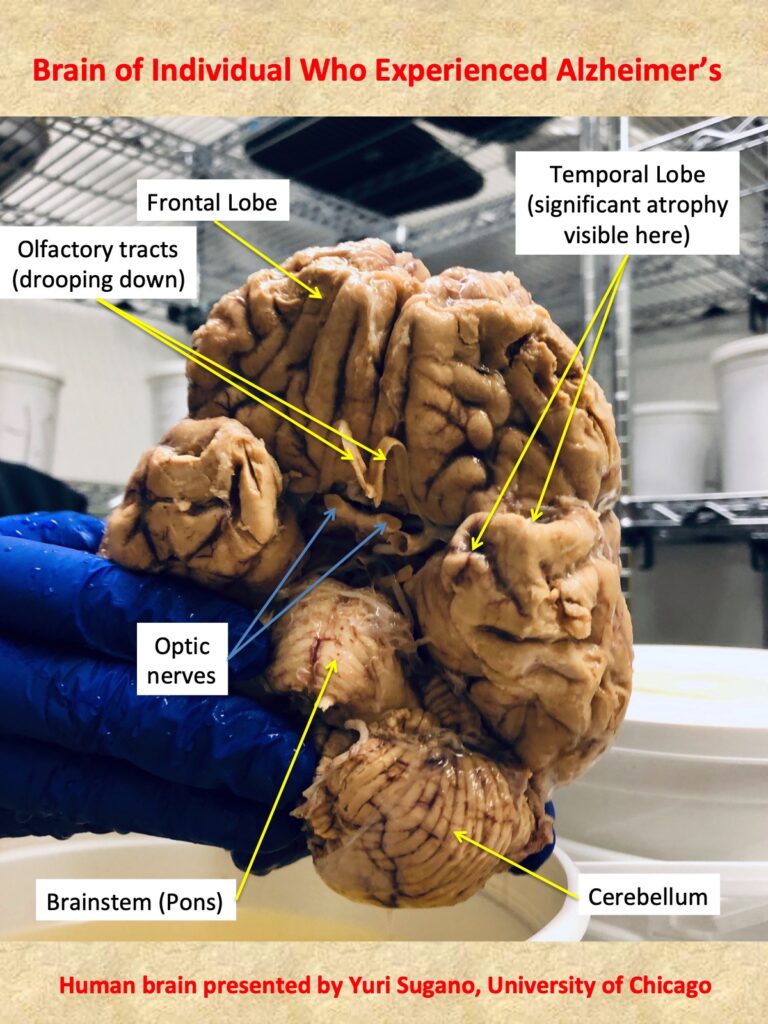
The neurophysiological underpinnings of the disease are starting to become more clear with more research being done. One mechanism of the disease has been found to be linked to the accumulation of fibrous plaques of a protein called ß-amyloid , or “amyloid–beta”, that aggregates around the neuron, impairing it to properly execute signaling along its pathways.
In theory, if the synthesis of ß-amyloid protein could be modified, this could restore some of the cognitive and behavioral impairments seen in AD. Alzheimers is the main focus in Dr. Grays Lab, and one of the most important neurodegenerative diseases I aim to understand.
LEARN MORE: Neuron-to-Neuron ß-amyloid Signaling in Neurodegeneration
LEARN MORE: Amyloid-beta neurotoxicity in Alzheimer’s Mice
LEARN MORE: ß-amyloid peptide and Neurofibrillary Tangles in AD Pathogenesis
Hands-on in the lab
After a few months, I went from feeling doubtful of even being left on my own, to performing an entire series of treatments, assays, and analyses confidently. Being able to try out things from growing frozen neurons in culture, to running assays I had only heard of in science journals, I’ve gained more knowledge about research this year than I ever have through a textbook.
It took me a while to fully understand what I was doing. Whenever I made an error, or didn’t understand a calculation, my science spirit suffered a little. I’m grateful that everyone in Dr. Gray’s lab is supportive and reassuring, which is why I was able to build my spirit up again after each trial and tribulation.
And there were many!
Let’s talk about Western Blots

Working in a lab means learned lab skills – and techniques!
A technique called Western Blot is used to analyze the amount and type of protein present in a sample. The amount of protein between different treatment groups may be indicative of a significant change in expression of that protein. If that protein is in charge of something like the “integrity of the cytoskeletal network” then the Western blot will show how much is being produced in each of the samples, relative to a standard. This is a highly simplified example. See the links below for more information!
Learning the Western Blot has been single handedly my toughest series of trials and tribulations so far in my science journey. Thankfully, I was comforted to hear from others in my lab that hardship on your first Western is almost like a rite of passage.
LEARN MORE: Western blotting
LEARN MORE: NCBI Western Blot Overview
LEARN MORE: SOLVED: Your Three Most Common Western Blotting Problems
LEARN MORE: 7 Tips to Improve Your Western Blot
Because I found the Western Blot so difficult, breaking it down in a digestible manner was vital for my understanding. A Western Blot involves three steps: gel electrophoresis, transferring the gel, and developing the membrane.
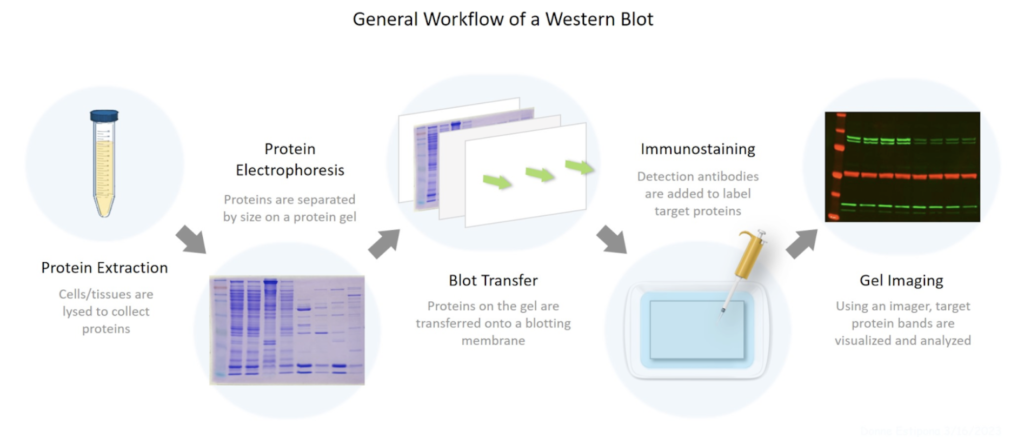
LEARN MORE: BioRad: Overview of Western Blot Procedure
LEARN MORE: What is Western Blotting?
Gel Electrophoresis
This is a technique that I was exposed to in my undergraduate science courses and is a fundamental technique used in many different sorts of analyses. Essentially, a chamber, which is connected to a voltage-powered machine, with a positive and negative end, drives a process that separates the proteins within each sample loaded into one end of the gel by their weight, size, and charge.

LEARN MORE: Western Blotting Sample Preparation
LEARN MORE: Western Blotting Animation Part I
LEARN MORE: Western Blotting Animation Part II
Transferring the gel
In order to transfer the gel onto a more usable and pliable medium, it must be transferred onto a thin membrane – essentially a paper. There are two methods to this transfer: slow transfer and fast. In our lab we use a semi-dry fast transfer which uses a power blotter to compress the gel and the membrane together to transfer the bands on the gel onto the membrane.
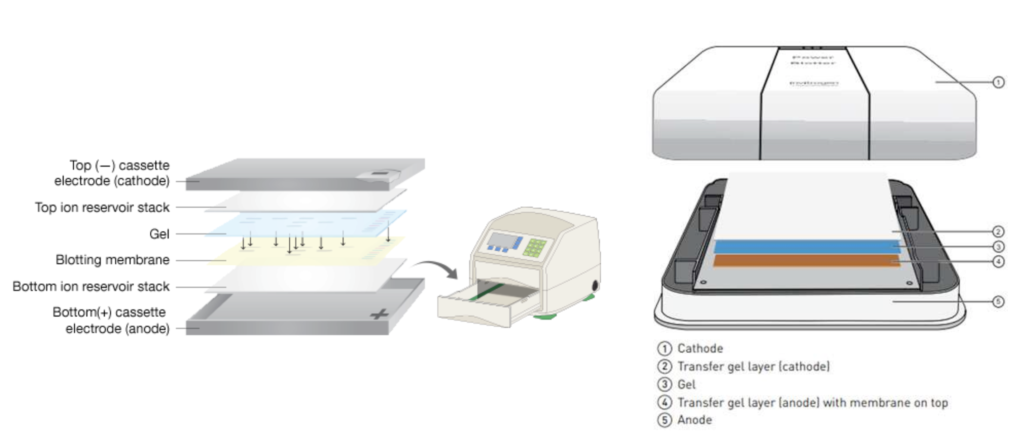
LEARN MORE: Efficient transfer of proteins from gel to membrane
After washing the membrane with a buffer such as Bovine Serum-Albumin (BS), and before imaging the membrane, the protein bands of interest have to be targeted using a primary and secondary antibody.
This process consists of incubating the membrane in the primary antibody from a few hours to a few days. Essentially, the membrane (which is the transferred image of the original gel, remember..), takes a bath to soak up the antibody.
The primary antibody (developed in a rabbit) binds to our protein of interest.

LEARN MORE: Immunoassays and Antibody Staining
After a few days in the primary antibody, the membrane must be exposed to a secondary antibody that will target the primary antibody, to visualize it and the protein it’s bound to.
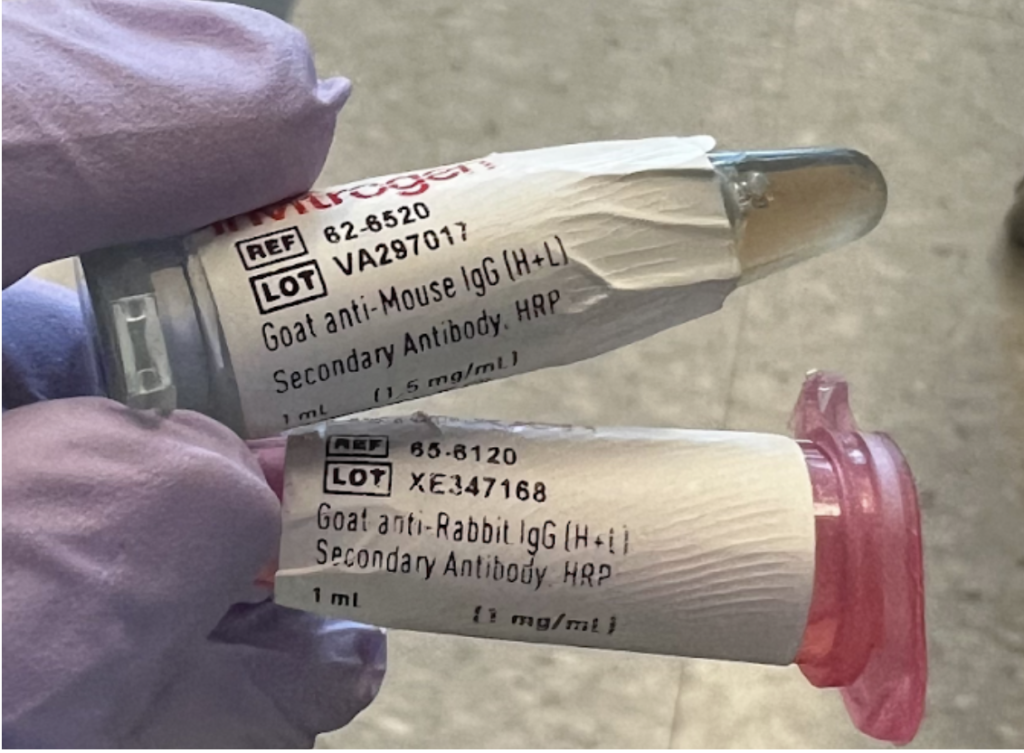
Notice there is only one species (rabbit) in the primary antibody (top picture), and two species in the secondary antibody (picture immediately above). The second species, in the pink microcentrifuge tube above must match the species of the first antibody (in this case “Rabbit”) in order to properly bind.
LEARN MORE: Importance of Secondary Antibody Reactivity, IgG class, and Conjugation
LEARN MORE: HRP and AP-conjugated Secondary Antibodies
Developing the membrane
After an hour or so in the secondary antibody, the membrane can be imaged using high-tech machinery that exposes the membrane to varying exposures of light. Eventually, allowing for a clear image of visible bands in the sample lanes of the membrane.

This is a 1/2 membrane in a sterile “sleeve,” saturated in chemiluminescent substrate, which will be placed in the imaging machine to be exposed.
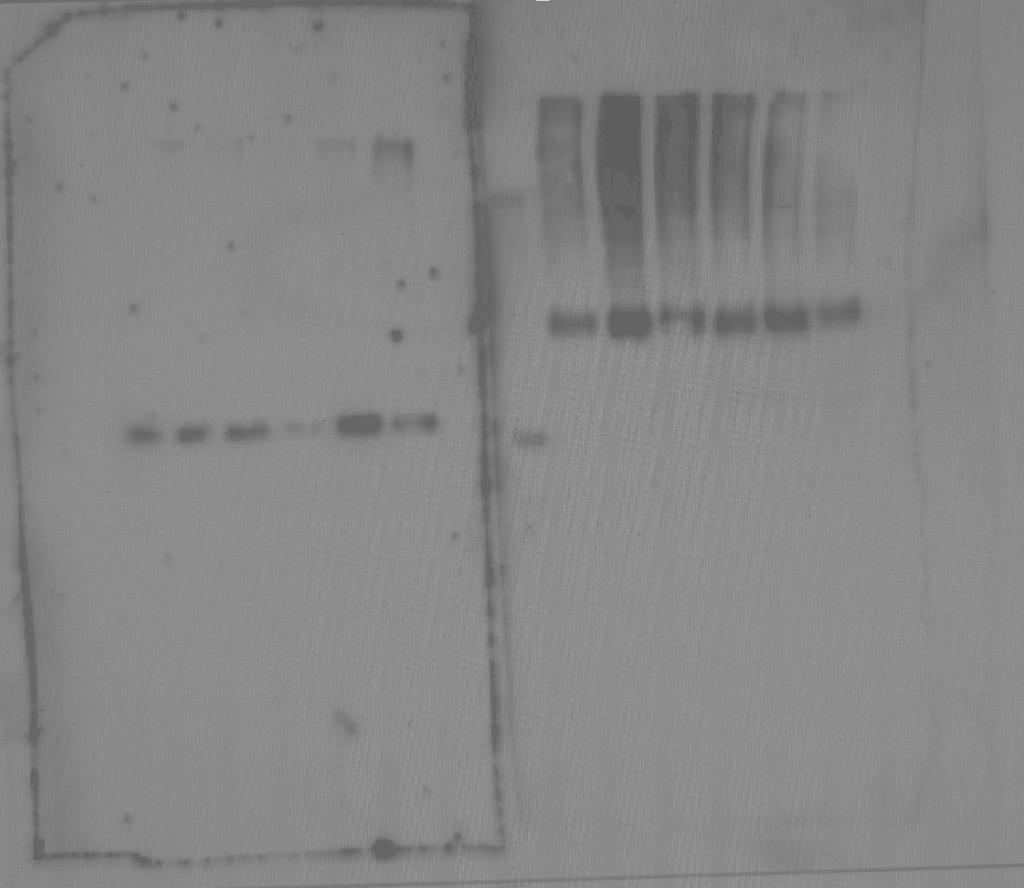
This is a membrane after imaging. These are two half membranes side by side, targeting different things: a loading control measurement (left) and the target antibody (right). The specific density of the bands can be determined with further analysis, helping to confirm your protein identity.
Trials and tribulations
Getting a proper image is where I ended up stuck for an entire term. It’s been a tumultuous journey. Trial-after-trial with no results almost made me reconsider my future as a scientist. I second-guessed myself constantly.
There are many points where a Western Blot can go South.
Being unsuccessful at Western Blot is basically a lab rite of passage. From my experience, the various areas where a Western Blot can go wrong are wide-ranging. Let’s start with where I went wrong.
It’s your cells.
I had originally been running my Western Blot on the same set of cell samples. In order to rule out whether or not my technique was at fault, I did a Western Blot on a new set of samples, executing the procedure in the exact same manner I had been monotonously. Thankfully, a result was produced.
It’s your antibody.
At another point, I came across another hurdle which was fixed by changing the secondary antibody to a slightly different form.

NOTE: Reusable antibody cocktails can become less potent after long term storage, so sodium azide (NaN3) is often added in to preserve their effectiveness.
LEARN MORE: Potential Problems with Western Blot Antibodies
LEARN MORE: Western Blot: Technique, Theory, and Trouble Shooting
It’s your technique or equipment.
If it’s not your cells, it may be your technique or equipment. In the case of contaminating my original cell samples, my technique while growing them originally could have been the sole cause. This can be ruled out by having someone who has successfully run that protocol, with those cells, do the experiment and see if the results match up.
LEARN MORE: NCBI Technique, Theory, and Troubleshooting in Western Blot
You didn’t “wash” your membranes enough.
Between antibodies, rinsing the membrane with a wash buffer that transfers any extra debris into the waste solution is important in order to properly visualize your target protein and reduce excess signaling.
If you didn’t rinse, you could end up with a very funky membrane image!
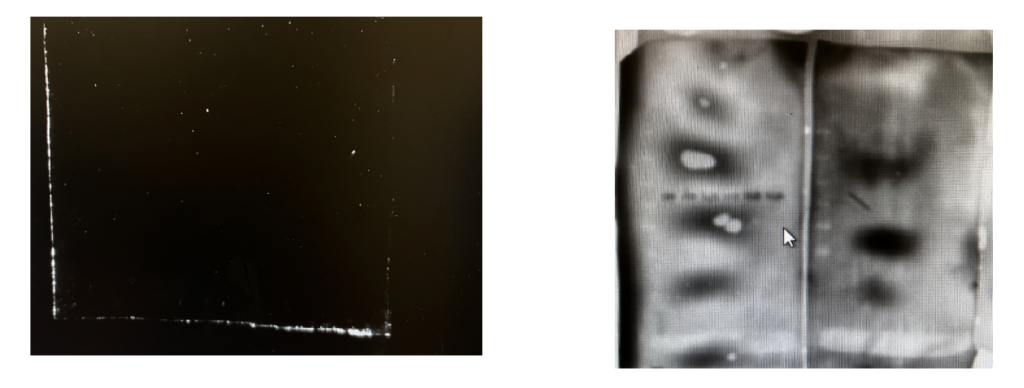
There are a ton of other reasons your Western Blot may not be working out, but that’s science. Mistakes matter, and are essential for discovery and improvement.
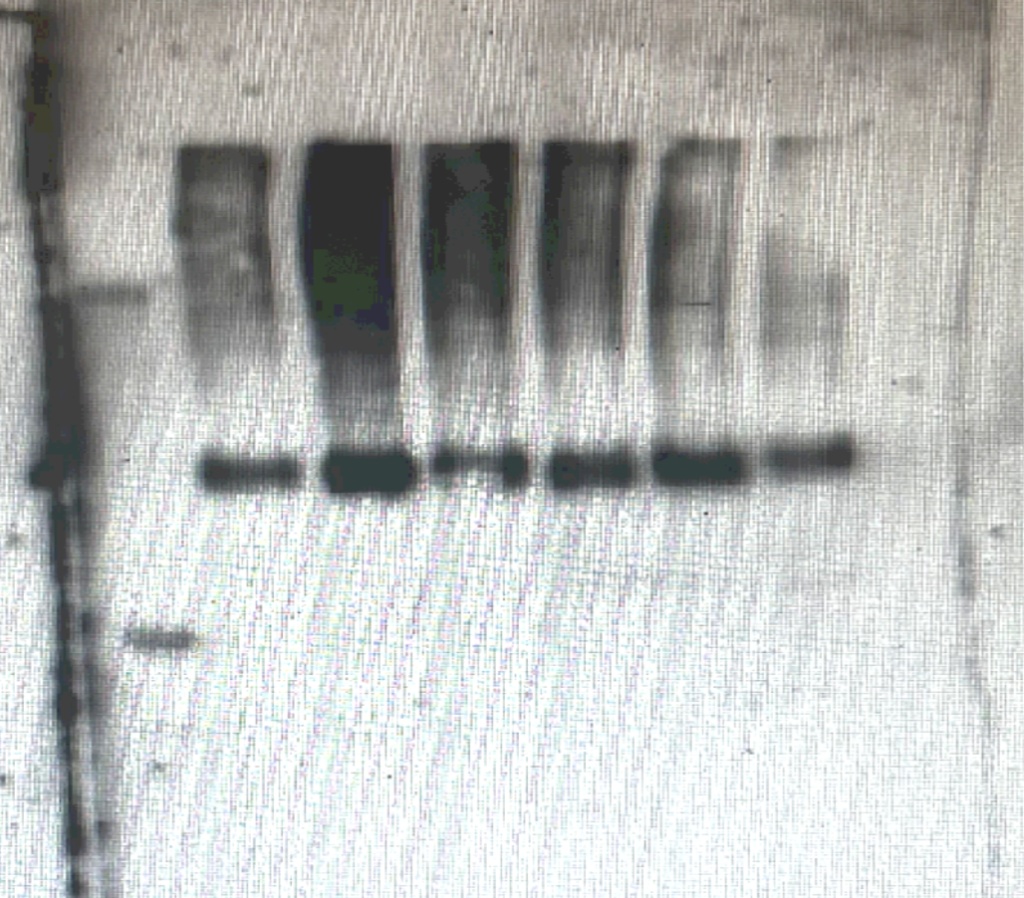
When my Westerns started working it was worth every hurdle.
LEARN MORE: Southern blotting
LEARN MORE: Southern blot
What undergraduate research has taught me
I never thought I would be doing what I’m now able to do in the lab so quickly.
Thankfully, my misconceptions and stereotypes about research began to topple as I grasped my footing in the lab. I would have never been able to acquire these opportunities without the help from BUILD Exito and my mentors along the way. My plans are to continue in Dr. Gray’s Lab until the foreseeable future. I can’t thank EXITO enough for matching me! I will be forever grateful for these opportunities, and I’m eager to see where my next steps in my science career lead to as I head off for graduation.